How Many Absorptions Would You Expect the Following Compound to Have:
Theory
An invaluable tool in organic structure determination and verification involves the class of electromagnetic (EM) radiation with frequencies between 4000 and 400 cm-1 (wavenumbers). The category of EM radiation is termed infrared (IR) radiation, and its application to organic chemistry known as IR spectroscopy. Radiation in this region can be utilized in organic structure determination by making use of the fact that it is absorbed by interatomic bonds in organic compounds. Chemical bonds in different environments will absorb varying intensities and at varying frequencies. Thus IR spectroscopy involves collecting absorption information and analyzing it in the form of a spectrum -- The frequencies at which there are absorptions of IR radiation ("peaks" or "signals") can be correlated directly to bonds within the compound in question.
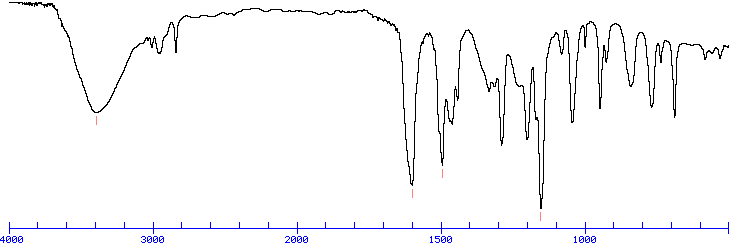
An Example IR Spectrum
Because each interatomic bond may vibrate in several different motions (stretching or bending), individual bonds may absorb at more than one IR frequency. Stretching absorptions usually produce stronger peaks than bending, however the weaker bending absorptions can be useful in differentiating similar types of bonds (e.g. aromatic substitution). It is also important to note that symmetrical vibrations do not cause absorption of IR radiation. For example, neither of the carbon-carbon bonds in ethene or ethyne absorb IR radiation.
Regions of the IR Spectrum
Over time organic chemists have recorded and catalogued the types and locations of IR absorptions produced by a wide variety of chemical bonds in various chemical environments. These data can be quickly referenced through tables of IR absorption ranges and compared to the spectrum under consideration. As a general rule, the most important factors determining where a chemical bond will absorb are the bond order and the types of atoms joined by the bond. Conjugation and nearby atoms shift the frequency to a lesser degree. Therefore the same or similar functional groups in different molecules will typically absorb within the same, specific frequency ranges. Consequently tables of IR absorptions are arranged by functional group -- it some versions these may be further subdivided to give more precise information.
In IR absorption tables, signal intensities (height) are usually denoted by the following abbreviations: w = weak, m = medium, s = strong, v = variable. A broad signal shape is sometimes indicated by br. Occasionally absorption frequency is given as a single approximation denoted with an ~ rather than a range.
A Table of IR Absorptions
These trends in aborption can be further summarized into the following categories
| 3600 - 2700 cm-1 | X-H |
| 2700 - 1900 cm-1 | X=Y |
| 1900 - 1500 cm-1 | X=Y |
| 1500 - 500 cm-1 | X-Y |
Upon first inspection, a typical infrared spectrum can be visually divided into two regions. The left half, above 2000 cm-1, usually contains relatively few peaks, but some very diagnostic information can be found here. First, alkane C-H stretching absorptions just below 3000 cm-1 demonstrate the presence of saturated carbons, and signals just above 3000 cm-1 demonstrate unsaturation. A very broad peak in the region between 3100 and 3600 cm-1 indicates the presence of exchangeable protons, typically from alcohol, amine, amide or carboxylic acid groups (see further discussion of this below). The frequencies from 2800 to 2000 cm-1 are normally void of other absorptions, so the presence of alkyne or nitrile groups can be easily seen here.
In contrast, the right half of the spectrum, below 2000 cm-1, normally contains many peaks of varying intensities, many of which are not readily identifiable. Two signals which can be seen clearly in this area is the carbonyl group, which is a very strong peak around 1700 cm-1, and the C-O bond with can be one or two strong peaks around 1200 cm-1. This complex lower region is also known as the "fingerprint region" because almost every organic compound produces a unique pattern in this area -- Therefore identity can often be confirmed by comparison of this region to a known spectrum.
Additional IR Concepts
Although the above and similar IR absorption tables provide a good starting point for assigning simple IR spectra, it is often necessary to understand in greater detail some more specific properties of IR spectra. The following topics cover some of the most important of these principles.
As mentioned previously, one of the major factors influencing the IR absorption frequency of a bond are the identity of the two atoms involved. To be more precise, it is the masses of the two atoms which are of greater importance. The greater the masses of attached atoms, the lower the IR frequency at which the bond will absorb. An example of this are the spectra of chloroform and deuterochloroform -- notice that the two major differences in these spectra are (1) the disappearance of the C-H stretching (3020 cm-1) and bending (1220 cm-1) in deuterated compound and (2) a shift to the right about 20 cm-1 relative to the CHCl3. The first is caused simply by the lack of C-H bonds in CDCl3. The second is illustrative of this property that heavier atoms (deuterium vs. hydrogen) will cause attached bonds to absorb at lower frequencies.
One of the most distinct and easily recognizable peaks in an IR spectrum is the broad O-H absorption of alcohols and phenols. However it important to understand why this broadening takes place and to consider the situations in which the peak may not have this characteristic shape. First, note that any significant quantity of a compound will contain a very large number of individual molecules, and each of these may be hydrogen bonded to a slightly different extent. Thus as an IR spectrum is acquired IR absorptions will occur at varying frequencies for each of these bonds. The end result is that the IR peak appears broadened, as it is an average of all these slightly different absorptions.
It is possible to acquire IR spectra of hydroxyl-containing compounds without seeing this broad signal. By creating a very dilute solution of the sample, or acquiring the IR spectra in the gas phase, hydrogen bonding is prevented through lack of molecular contact. Even in concentrated solution, larger compounds may sterically hinder hydrogen bonding, preventing exchange. In these situations the broad O-H peak is replaced by a sharp signal around 3600 cm-1. This effect can be seen in the IR spectra of t-butanol, dilute and concentrated.
How Many Absorptions Would You Expect the Following Compound to Have:
Source: https://webspectra.chem.ucla.edu/irintro.html